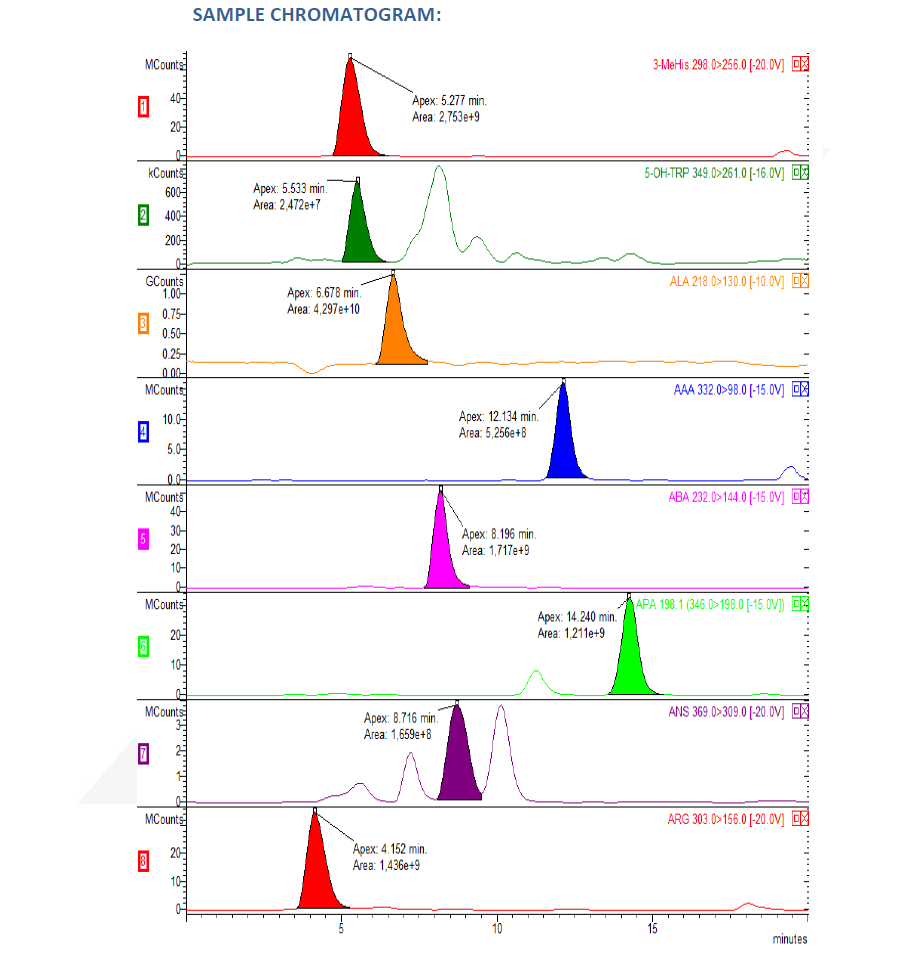
Introduction
LC-MS/MS is a powerful tool for analyzing amino acid profiles in fluids like blood or urine. This information is critical for diagnosing various inborn errors of metabolism (IEMs) and other conditions affecting amino acid balance. Commercially available kits designed for LC-MS/MS can simplify workflows in labs. However, using these kits for diagnosing patients requires following specific regulations.
Regulations by Region
The rules for using amino acid analysis kits vary depending on location. Here's a look at some key regions:
- United States (US): The FDA categorizes medical testing tools (in vitro diagnostics or IVDs) based on their risk. Kits used to diagnose IEMs would likely be Class II or Class III, requiring approval through specific procedures before being sold.
- European Union (EU): The IVDR regulation governs IVDs in the EU. Similar to the US system, the risk classification of the kit determines the approval process. High-risk devices may require additional assessments.
- Other Regions: Regulations in other countries may differ. Consulting the relevant health agency is essential.
Important Considerations for Clinical Use
Beyond regulatory approval, several other factors are important when considering amino acid analysis kits for diagnosing patients:
- Analytical Validation: The kit's accuracy, precision, and ability to detect specific levels of amino acids need to be thoroughly tested following established guidelines.
- Clinical Validation: Studies are needed to show the kit's effectiveness in diagnosing specific conditions in real patients.
- Quality Control (QC): A strong QC program ensures consistent and reliable results. This includes using specific controls, monitoring performance, and having procedures to fix problems.
- User Training: Personnel performing the tests must be properly trained on using the kit, interpreting results, and understanding its limitations.
Conclusion
Amino acid analysis kits using LC-MS/MS offer significant advantages for clinical research and potentially for routine patient testing. However, navigating the regulatory landscape and ensuring proper validation and implementation are crucial for reliable and legal use. Researchers and clinicians should stay updated on relevant regulations and best practices to ensure accurate and valuable information for patient care.